S.I. No. 33/2019 - European Communities (Quality And Safety Of Human Tissues And Cells) (Amendment) Regulations 2019
Notice of the making of this Statutory Instrument was published in | ||
“Iris Oifigiúil” of 12th February, 2019 | ||
I, Simon Harris, Minister for Health, in exercise of the powers conferred on me by section 3 of the European Communities Act 1972 (No. 27 of 1972) and for the purpose of giving effect to Commission Directive (EU) 2015/5661 of 8 April 2015 implementing Directive 2004/23/EC as regards the procedures for verifying the equivalent standards of quality and safety of imported tissues and cells, hereby make the following regulations: | ||
PART 1 | ||
PRELIMINARY AND GENERAL | ||
Citation | ||
1. These Regulations may be cited as the European Communities (Quality and Safety of Human Tissues and Cells) (Amendment) Regulations 2019. | ||
Definition | ||
2. In these Regulations, “Principal Regulations” means the European Communities (Quality and Safety of Human Tissues and Cells) Regulations 2006 ( S.I. No. 158 of 2006 ). | ||
PART 2 | ||
AMENDMENT OF PRINCIPAL REGULATIONS | ||
Amendment of Regulation 2 of Principal Regulations | ||
3. Regulation 2 (1) of the Principal Regulations is amended - | ||
(a) by the insertion of the following definitions: | ||
“ ‘emergency’ means any unforeseen situation in which there is no practical alternative other than to urgently import tissues and cells from a third country into the European Union for immediate application to a known recipient or known recipients whose health would be seriously endangered without such an import; | ||
‘importing activity’ means any activity consisting of any aspect of - | ||
(a) the importation of human tissues and cells intended for human application into the European Union; | ||
(b) the importation of manufactured products derived from human tissues and cells intended for human application, where those products are not covered by other European Union legislation; | ||
(c) the importation of human tissues and cells which are intended to be used exclusively in manufactured products which are covered by other European Union legislation; | ||
‘importing tissue establishment’ means a tissue bank or a unit of a hospital or another body established within the European Union which is a party to a contractual agreement with a third country supplier for the import into the European Union of tissues and cells coming from a third country intended for human application; | ||
‘Imported Tissues and Cells Directive’ means Commission Directive (EU) 2015/566 of 8 April 20152 implementing Directive 2004/23/EC as regards the procedures for verifying the equivalent standards of quality and safety of imported tissues and cells; | ||
‘one-off import’ means the import of any specific type of tissue or cell which is for the personal use of an intended recipient or recipients known to the importing tissue establishment and the third country supplier before the importation occurs, and such an import of any specific type of tissue or cell shall normally not occur more than once for any given recipient but shall not include imports from the same third country supplier taking place on a regular or repeated basis; | ||
‘third country supplier’ means a tissue establishment or another body, established in a third country, which is responsible for the export to the European Union of tissues and cells it supplies to an importing tissue establishment, which supplier may also carry out one or more of the activities, which take place outside of the European Union, of donation, procurement, testing, processing, preservation, storage or distribution of tissues and cells imported into the European Union;”, | ||
(b) in the definition of “site”, by the substitution of “any prescribed activity or activities or importing activity or activities” for “any prescribed activity or activities”, | ||
(c) by the substitution of the following definition for the definition of “the Directives”: | ||
“ ‘the Directives’ means the Directive and Commission Directive 2006/17/EC of 8 February 20063 , as amended by Commission Directive 2012/39/EU of 26 November 20124 , Commission Directive (EU) 2015/565 of 8 April 20155 amending Directive 2006/86/EC as regards certain technical requirements for the coding of human tissues and cells and the Imported Tissues and Cells Directive;”, and | ||
(d) by the substitution of the following definition for the definition of “responsible person”: | ||
“ ‘responsible person’, in relation to a tissue establishment or an importing tissue establishment, means the person who has been designated pursuant to Regulation 8 as the responsible person for that tissue establishment or importing tissue establishment, as the case may be;”. | ||
Amendment of Regulation 3 of Principal Regulations | ||
4. Regulation 3 of the Principal Regulations is amended - | ||
(a) by the substitution of the following paragraph for paragraph (1): | ||
“(1) Subject to paragraphs (2) and (2A), these Regulations shall apply to all prescribed activities and to all importing activities.”, | ||
(b) in paragraph (2) – | ||
(i) in subparagraph (c), by the substitution of “human body;” for “human body”, and | ||
(ii) by the insertion of the following subparagraph after subparagraph (c): | ||
“(d) the import of tissues and cells in accordance with Regulation 15(4)(a) or (b);”, | ||
(c) by the insertion of the following paragraph after paragraph (2): | ||
“(2A) Where the human tissues and cells to be imported are intended to be used exclusively in manufactured products which are covered by other European Union legislation, the Imported Tissues and Cells Directive shall only apply to the donation, procurement and testing which takes place outside of the European Union as well as to contributing to ensuring traceability from donor to recipient and vice versa.”, and | ||
(d) by the substitution of the following paragraph for paragraph (4): | ||
“(4) These Regulations shall apply without prejudice to the Data Protection Acts 1988 to 2018.” | ||
Amendment of Regulation 5 of Principal Regulations | ||
5. Regulation 5 of the Principal Regulations is amended - | ||
(a) in paragraph (1), by the substitution of “any prescribed activity or any importing activity” for “any prescribed activity”, and | ||
(b) in paragraph (2), by the substitution of “Tissue establishments and importing tissue establishments” for “Tissue establishments”. | ||
Amendment of Regulation 6 of Principal Regulations | ||
6. Regulation 6 of the Principal Regulations is amended - | ||
(a) by the substitution of the following paragraph for paragraph (1): | ||
“(1) Subject to Regulation 6(2), the Health Products Regulatory Authority may grant an authorisation to - | ||
(a) a tissue establishment to carry out any prescribed activity at a specified site or sites, having satisfied itself that the tissue establishment - | ||
(i) complies with the requirements referred to in Article 28(a) of the Directive, and | ||
(ii) complies with other relevant requirements of the Directives and these Regulations, or | ||
(b) an importing tissue establishment to carry out any importing activity at a specified site or sites, having satisfied itself that the importing tissue establishment - | ||
(i) complies with the requirements referred to in Article 28(a) of the Directive, | ||
(ii) complies with the requirements referred to in Article 5(1) of the Imported Tissues and Cells Directive, and | ||
(iii) complies with other relevant requirements of the Directives and these Regulations.”, | ||
(b) by the insertion of the following paragraphs after paragraph (1) - | ||
“(1A) Subject to paragraph (1B) an importing tissue establishment shall provide the documentation in Schedule 8 to the Health Products Regulatory Authority when requested. | ||
(1B) Paragraph (1A) shall not apply to importing tissue establishments in respect of one-off imports provided - | ||
(a) the responsible person of the importing tissue establishment notifies the Health Products Regulatory Authority in writing, prior to the one-off importation of tissues or cells specifying - | ||
(i) the type of tissues or cells to be imported, | ||
(ii) the name and address of the tissue establishment or organisation from which the tissues or cells are to be imported, and | ||
(iii) the tissues or cells code or reference number, | ||
(b) the responsible person of the importing tissue establishment provides evidence to the Health Products Regulatory Authority that confirms that the tissues or cells which are to be imported are tested in conformity with the testing requirements for tissues and cells specified in these Regulations, including any additional tests which may be necessary for specific tissues or cells, types of donors or epidemiological situations, | ||
(c) imported tissues and cells are applied to their intended recipients, and | ||
(d) a list of the activities carried out by the third country supplier is supplied by the importing tissue establishment to the Health Products Regulatory Authority prior to import.”, | ||
(c) by the substitution, in paragraph (4), of “Subject to paragraph (4A), all applications” for “All applications”, | ||
(d) by the insertion of the following paragraph after paragraph (4): | ||
“(4A) An application by an importing tissue establishment shall - | ||
(a) include- | ||
(i) the required information and documentation as set out in Schedule 6, | ||
(ii) a copy of any written agreement made between the importing tissue establishment and a third country supplier under Regulation 16A(1), and | ||
(iii) any other relevant information as determined by the Health Products Regulatory Authority, and | ||
(b) be accompanied by the appropriate fee.”, | ||
(e) in paragraph (5), by the substitution of the following subparagraph for subparagraph (b): | ||
“(b) grant such authorisation - | ||
(i) to tissue establishments in respect of particular sites or prescribed activities only, | ||
(ii) to importing tissue establishments in respect of particular sites or importing activities only, and | ||
(iii) subject to conditions.”, | ||
(f) by the substitution of the following paragraph for paragraph (6): | ||
“(6) Subject to paragraph (6A), where the Health Products Regulatory Authority grants an authorisation, it shall give notice in writing to - | ||
(a) the tissue establishment specifying, in the case of prescribed activities, the prescribed activities which the tissue establishment may undertake under these Regulations at each site in respect of which authorisation is granted, and if the grant is subject to conditions, the conditions which apply to the undertaking of those activities, or | ||
(b) the importing tissue establishment specifying, in the case of importing activities, the importing activities which the importing tissue establishment may undertake under these Regulations at each site in respect of which authorisation is granted, and if the grant is subject to conditions, the conditions which apply to the undertaking of those activities.”, | ||
(g) by the insertion of the following paragraph after paragraph (6): | ||
“(6A) Where the Health Products Regulatory Authority grants an authorisation under paragraph (5) to an importing tissue establishment, it shall issue the establishment with a certificate as set out in Schedule 7.”, | ||
(h) in paragraph (8), by the substitution of “on the tissue establishment or the importing tissue establishment concerned as the case may be” for “on the tissue establishment concerned”, | ||
(i) by the insertion of the following paragraph after paragraph (9): | ||
“(9A) An importing tissue establishment shall not make any substantial changes in the importing activities it undertakes without the prior written approval of the Health Products Regulatory Authority.”, | ||
(j) by the substitution, in paragraph (10), of “a tissue establishment or importing tissue establishment” for “a tissue establishment”, and | ||
(k) by the insertion of the following paragraph after paragraph (11): | ||
“(11A) (a) For the purpose of an importing tissue establishment, a substantial change shall include any changes to - | ||
(i) the type of tissues and cells imported, | ||
(ii) the activities undertaken in third countries which may have an influence on the quality and safety of imported tissues and cells, | ||
(iii) the third country suppliers used, | ||
(iv) the site or sites from which the importing tissue establishment operates or to the importing activities to be carried out at each site, which would result in a failure to comply with the requirements of the Directives and these Regulations, or | ||
(v) the quality system, as set out in accordance with Article 28(c) of the Directive, which is likely to have a substantial impact on the conduct of, or might compromise the safety of, any of the importing activities which the importing tissue establishment has been authorised to undertake pursuant to this Regulation. | ||
(b) Where an importing tissue establishment undertakes a one-off import of tissues or cells originating from a third country supplier not covered by its existing authorisation, such an import shall not be considered as a substantial change for the purposes of paragraph (9A), if the importing tissue establishment is authorised to import the same type of tissues or cells from another third country supplier or suppliers.”. | ||
Suspension or revocation of authorisation | ||
7. The Principal Regulations are amended by the substitution of the following Regulation for Regulation 7: | ||
“7. (1) Subject to paragraph (2), the Health Products Regulatory Authority may suspend or revoke the authorisation of a tissue establishment or of an importing tissue establishment in respect of a site or sites or prescribed activity or importing activity, as the case may be, on one or more of the following grounds: | ||
(a) that the tissue establishment, or importing tissue establishment, as the case may be, or the tissue and cell preparation process has not complied with the requirements of the Directives and these Regulations; | ||
(b) that a prescribed activity, or importing activity, as the case may be, has not been or cannot be carried out pursuant to the requirements of the Directives and these Regulations; | ||
(c) that any tissues or cells cannot be supplied to hospitals for human application in such a state that they could be safely used; | ||
(d) that the information given by the tissue establishment pursuant to Regulation 6(4) and 19(3) was false or incomplete in any material respect; | ||
(e) that the information given by the importing tissue establishment pursuant to Regulation 6(4), 6(4A) and 19(3) was false or incomplete in any material respect. | ||
(2) Subject to paragraph (3), before suspending or revoking the authorisation of a tissue establishment or of an importing tissue establishment, the Health Products Regulatory Authority shall serve notice on the tissue establishment, or the importing tissue establishment as the case may be, stating that it intends to suspend or revoke its authorisation with effect from the date specified in the notice which shall be not less than 7 days from the date on which the notice is served. | ||
(3) Where the Health Products Regulatory Authority considers that it is necessary in the interests of safety, it may, by notice served on a tissue establishment, or an importing tissue establishment as the case may be, suspend or revoke its authorisation with immediate effect. | ||
(4) Where - | ||
(a) the tissue establishment, or the importing tissue establishment as the case may be, has failed, in any material respect, to comply with the requirements of these Regulations, or | ||
(b) the information given by the tissue establishment pursuant to Regulation 6(4) and 19(3) was false or incomplete in any material respect, or | ||
(c) the information given by the importing tissue establishment pursuant to Regulation 6(4), 6(4A), and 19(3) was false or incomplete in any material respect, | ||
and the Health Products Regulatory Authority considers that the failure in question is not sufficiently serious to warrant suspension or revocation of the authorisation of the tissue establishment, or of the importing tissue establishment as the case may be, in the first instance, it may serve a notice on the responsible person of the tissue establishment, or on the responsible person of the importing tissue establishment, as the case may be, in accordance with paragraph (5). | ||
(5) A notice served under this paragraph shall - | ||
(a) identify the requirements of these Regulations in respect of which the tissue establishment, or the importing tissue establishment as the case may be, has failed to comply with or, in the case of false or incomplete information, the further information which is required, | ||
(b) identify the action which the tissue establishment, or the importing tissue establishment as the case may be, is required to take, and | ||
(c) give the timescale within which the tissue establishment, or the importing tissue establishment as the case may be, shall take the action identified in subparagraph (b). | ||
(6) If the tissue establishment, or the importing tissue establishment as the case may be, fails to comply with the requirements set out in the notice within the specified timescale, the Health Products Regulatory Authority may, by notice served on the tissue establishment, or on the importing tissue establishment as the case may be, suspend or revoke the authorisation of the tissue establishment, or of the importing tissue establishment. | ||
(7) A suspension or revocation pursuant to paragraph (6) shall take effect - | ||
(a) in a case where the Health Products Regulatory Authority considers that it is necessary in the interests of safety, immediately, or | ||
(b) in all other cases, from a date specified in the notice. | ||
(8) Any suspension pursuant to paragraph (1) or (6) shall be for such period as the Health Products Regulatory Authority considers necessary having regard to the reasons for the suspension. | ||
(9) The suspension or revocation of an authorisation under paragraph (1) or (6) may be total, or may be limited to: | ||
(i) in the case of a tissue establishment, a particular prescribed activity or to one or more prescribed activities carried out at a particular site or sites; | ||
(ii) in the case of an importing tissue establishment, a particular importing activity or to one or more importing activities carried out at a particular site or sites; | ||
(iii) in the case of either a tissue establishment or an importing tissue establishment, a particular tissue or cell.”. | ||
| ||
Responsible person for tissue establishment | ||
8. The Principal Regulations are amended by the substitution of the following Regulation for Regulation 8: | ||
“8. (1) Subject to paragraph (2), a tissue establishment, or an importing tissue establishment as the case may be, shall designate a person who is responsible for the following: | ||
(a) ensuring that all prescribed activities, or importing activities, as the case may be, are carried out in accordance with the requirements of these Regulations; | ||
(b) providing information as required under Regulation 6 to the Health Products Regulatory Authority; | ||
(c) in the case of a tissue establishment, the implementation in that tissue establishment of the requirements under Regulations 9, 10, 11, 12, 14, 15, 16, 18, 19 and 20; | ||
(d) in the case of an importing tissue establishment, the implementation in that importing tissue establishment of the requirements under Regulations 9, 10, 11, 12, 14, 15,16,16A, 16B, 18, 19 and 20. | ||
(2) A tissue establishment, or an importing tissue establishment, as the case may be, shall not designate a person under paragraph (1) unless that person has - | ||
(a) a diploma, certificate or other evidence of formal qualification in the field of medical or biological sciences awarded on completion of - | ||
(i) a university course of study, or | ||
(ii) a course recognised as an equivalent course by the Health Products Regulatory Authority, and | ||
(b) practical post-graduate experience in areas of work relevant to the responsibilities of the responsible person under these Regulations for at least 2 years, in an establishment (or more than one establishment) in any Member State lawfully undertaking activities related to the collection or testing (or both) of tissues and cells, or to their procurement, storage and distribution. | ||
(3) The Health Products Regulatory Authority shall from time to time publish details of courses recognised by it for the purpose of paragraph (2)(a)(ii). | ||
(4) A tissue establishment or an importing tissue establishment shall inform the Health Products Regulatory Authority of the name of the responsible person referred to in paragraph (1). | ||
(5) The responsible person may delegate any of the functions specified in paragraph (1) to other persons who shall be qualified by training and experience to perform them. | ||
(6) A tissue establishment or an importing tissue establishment shall notify the Health Products Regulatory Authority of the name of any persons to whom functions have been delegated by the responsible person under paragraph (5), and the specific functions which have been delegated to such persons. | ||
(7) Where the responsible person or a person to whom functions have been delegated under paragraph (5) is permanently or temporarily replaced, the tissue establishment, or the importing tissue establishment, as the case may be, shall without delay provide the Health Products Regulatory Authority with the name of the replacement, details of his or her qualifications and the date on which the replacement began his or her duties. | ||
(8) If the Health Products Regulatory Authority considers that the responsible person does not meet the requirements of paragraph (2), it may serve a notice to that effect on the tissue establishment, or the importing tissue establishment as the case may be. | ||
(9) If, within 14 days of receiving a notice in accordance with paragraph (8), a tissue establishment, or an importing tissue establishment as the case may be, is not able to demonstrate to the reasonable satisfaction of the Health Products Regulatory Authority that the responsible person does meet the requirements of paragraph (2), it shall, without delay - | ||
(a) relieve him or her of the duties of responsible person in respect of the tissue establishment or the importing tissue establishment as the case may be, | ||
(b) appoint a new responsible person in his or her place, and | ||
(c) notify the Health Products Regulatory Authority that it has appointed a new responsible person and provide details of the name and qualifications of the person appointed.”. | ||
Amendment of Regulation 9 of Principal Regulations | ||
9. Regulation 9 of the Principal Regulations is amended - | ||
(a) by the substitution of the following paragraphs for paragraph (1): | ||
“(1) Tissue establishments and importing tissue establishments shall ensure that tissue and cell procurement and testing - | ||
(a) take place in conditions authorised for that purpose, and | ||
(b) are carried out by persons with appropriate training and expertise. | ||
(1A) Tissue establishments shall ensure that the tests required for donors shall be carried out by a qualified laboratory authorised by the Health Products Regulatory Authority.”, | ||
(b) in paragraph (2), by the substitution of “Tissue establishments and importing tissue establishments” for “The IMB”, and | ||
(c) by the insertion of the following paragraph after paragraph (3): | ||
“(3A) An importing tissue establishment shall ensure that appropriate control measures are in place for the procurement of tissues and cells in third countries.”. | ||
Amendment of Regulation 10 of Principal Regulations | ||
10. Regulation 10 of the Principal Regulations is amended - | ||
(a) in paragraph (1), by the substitution of “A tissue establishment or an importing tissue establishment, as the case may be,” for “A tissue establishment”, | ||
(b) in paragraph (2), by the substitution of “Tissue establishments and importing tissue establishments” for “Tissue establishments”, | ||
(c) in paragraph (3), by the substitution of “Tissue establishments and importing tissue establishments” for “Tissue establishments”, | ||
(d) in paragraph (4) - | ||
(i) by the substitution of “Tissue establishments and importing tissue establishments” for “Tissue establishments”, | ||
(ii) in subparagraph (b), by the substitution of “procured, imported, tested” for “procured, tested”, and | ||
(e) in paragraph (5), by the substitution of “storage, importation or distribution” for “storage or distribution”. | ||
Updated information | ||
11. The Principal Regulations are amended by the insertion of the following Regulation after Regulation 10: | ||
“10A. (1) An importing tissue establishment shall seek the prior written approval of the Health Products Regulatory Authority for any planned substantial changes to its import activities, in particular the substantial changes referred to in Regulation 6 (11A), and inform the Health Products Regulatory Authority of its decision to cease its import activities in part or in full. | ||
(2) An importing tissue establishment shall notify the Health Products Regulatory Authority, without delay, of any suspected or actual serious adverse events or reactions, reported to it by third country suppliers which may influence the quality and safety of the tissues and cells it imports. | ||
(3) A notification referred to in paragraph (2) shall include the information required by Schedules 3 and 4 to the European Communities (Human Tissues and Cells Traceability Requirements, Notification of Serious Adverse Reactions and Events and Certain Technical Requirements) Regulations 2007 ( S.I. No. 598 of 2007 ). | ||
(4) An importing tissue establishment shall notify the Health Products Regulatory Authority, without delay, of - | ||
(a) any revocation or suspension, in part or full, of a third country supplier’s authorisation to export tissues and cells, and | ||
(b) any other decision taken for reasons of non-compliance by the competent authority or authorities of the country in which the third country supplier is based which may be relevant to the quality and safety of imported tissues and cells.”. | ||
Amendment of Regulation 11 of Principal Regulations | ||
12. Regulation 11 of the Principal Regulations is amended - | ||
(a) in paragraph (1), by the substitution of “Tissue establishments and importing tissue establishments” for “The IMB and tissue establishments”, and | ||
(b) in paragraph (2), by the substitution of “Tissue establishments and importing tissue establishments” for “The IMB and tissue establishments”. | ||
Amendment of Regulation 12 of Principal Regulations | ||
13. Regulation 12 of the Principal Regulations is amended - | ||
(a) in paragraph (1), by the substitution of “Tissue establishments and importing tissue establishments” for “Tissue establishments”, | ||
(b) in paragraph (2), by the substitution of “tissue establishment, or the importing tissue establishment as the case may be,” for “tissue establishment,”, | ||
(c) by the substitution of the following paragraph for paragraph (3): | ||
“(3) Every tissue establishment and importing tissue establishment shall comply with the requirements set out in Schedule 4.”, | ||
(d) in paragraphs (5), (6), (7) and (8), by the substitution of “Tissue establishments and importing tissue establishments” for “Tissue establishments” in each place that it occurs, | ||
(e) by the substitution of the following paragraph for paragraph (9): | ||
“(9) Tissue establishments and importing tissue establishments shall have agreements and procedures in place to ensure that, in the event of termination of activities for whatever reason, stored tissues and cells shall be transferred to other tissue establishment or establishments, or if appropriate to other importing tissue establishment or establishments, authorised in accordance with Regulation 6 without prejudice to any domestic law concerning the disposal of donated tissues or cells, according to the consent pertaining to them.”, | ||
(f) by the substitution of the following paragraph for paragraph (10): | ||
“(10) Tissue establishments and importing tissue establishments shall ensure that personnel directly involved in activities relating to the procurement, importing, processing, preservation, storage and distribution of tissues and cells shall be qualified to perform such tasks and shall be provided with the training referred to in Article 28 (c) of the Directive.”, and | ||
(g) in paragraph (11), by the substitution of “Tissue establishments and importing tissue establishments” for “Tissue establishments”. | ||
Amendment of Regulation 13 of Principal Regulations | ||
14. Regulation 13 of the Principal Regulations is amended, in paragraph (3), by the substitution of “Tissue establishments and importing tissue establishments” for “Tissue establishments”. | ||
Amendment of Regulation 14 of Principal Regulations | ||
15. Regulation 14 of the Principal Regulations is amended, in paragraphs (3), (4) and (5), by the substitution of “Tissue establishments and importing tissue establishments” for “A tissue establishment” in both places that it occurs. | ||
Amendment of Regulation 15 of Principal Regulations | ||
16. Regulation 15 of the Principal Regulations is amended - | ||
(a) by the substitution of the following paragraph for paragraph (1): | ||
“(1) The Health Products Regulatory Authority shall ensure that - | ||
(a) all imports of tissues and cells from third countries are undertaken by authorised importing tissue establishments, and | ||
(b) all exports of tissues and cells to third countries are undertaken by authorised tissue establishments.”, | ||
(b) in paragraph (2), by the substitution of “An Importing tissue establishment” for “Tissue establishments”, and | ||
(c) by the substitution of the following paragraphs for paragraph (5): | ||
“(5) An importing tissue establishment shall take all necessary measures to ensure that imports of tissues and cells at paragraph (4) meet quality and safety standards equivalent to those laid down in the Directives and these Regulations. | ||
(5A) A tissue establishment shall take all necessary measures to ensure that exports of tissues and cells at paragraph (4) meet quality and safety standards equivalent to those laid down in the Directives and these Regulations.”. | ||
Amendment of Regulation 16 of Principal Regulations | ||
17. Regulation 16 of the Principal Regulations is amended - | ||
(a) by the substitution of the following paragraph for paragraph (1): | ||
“(1) Tissue establishments and importing tissue establishments shall establish written agreements with a third party each time an external activity takes place which influences the quality and safety of tissues and cells processed in cooperation with a third party, and in particular in the following circumstances: | ||
(a) where a tissue establishment entrusts one of the stages of tissue or cell processing to a third party; | ||
(b) where a third party provides goods and services that affect tissue or cell quality and safety assurance, including their distribution; | ||
(c) where a tissue establishment, or an importing tissue establishment, as the case may be, provides services to that establishment which is not authorised; | ||
(d) where a tissue establishment, or an importing tissue establishment as the case may be, distributes tissue or cells processed by third parties.”, | ||
(b) by the insertion of the following paragraph after paragraph (2): | ||
“(2A) Agreements between importing tissue establishments and third parties must be examined by the Health Products Regulatory Authority, within the authorisation framework of Regulation 6.”, | ||
(c) in paragraphs (3), (4) and (6), by the substitution of “Tissue establishments and importing tissue establishments” for “Tissue establishments” in each place where it occurs, and | ||
(d) by the insertion of the following paragraph after paragraph (5): | ||
“(5A) Agreements between importing tissue establishments and third parties shall specify the responsibilities of third parties and detailed procedures.”. | ||
Written agreements for importing tissue establishments | ||
18. The Principal Regulations are amended by the insertion of the following Regulations after Regulation 16: | ||
“16A. (1) Subject to paragraph (2), an importing tissue establishment shall have a written agreement in place with a third country supplier where any of the activities of donation, procurement, testing, processing, preservation, storage or export to the European Union of tissues and cells to be imported into the European Union are carried out outside the European Union. | ||
(2) Paragraph (1) shall not apply in relation to one-off imports of tissues and cells, provided that the importing tissue establishment concerned complies with Regulation 6(1B). | ||
(3) A written agreement referred to in paragraph (1) shall - | ||
(a) specify the quality and safety requirements to be met to ensure the equivalency of the quality and safety standards of the tissues and cells to be imported with the standards provided for in these Regulations, | ||
(b) include the contents listed in Schedule 9 and such other matters as the importing tissue establishment considers appropriate, and | ||
(c) provide that the Health Products Regulatory Authority may inspect the activities, including the facilities, of any third country suppliers during the duration of the written agreement and for a period of 2 years following its termination. | ||
Register of importing tissue establishments | ||
16B. (1) An importing tissue establishment shall keep a record of its activities for a period of not less than 30 years, including - | ||
(a) the types and quantities of tissues and cells imported, and | ||
(b) the origin and destination of tissues and cells imported. | ||
(2) A record referred to in paragraph (1) shall include the same information for any one-off imports carried out. | ||
(3) An importing tissue establishment shall include the information referred to in paragraph (1) in the annual report submitted to the Health Products Regulatory Authority under Regulation 10(4)(c). | ||
(4) The Health Products Regulatory Authority shall include importing tissue establishments in the publicly accessible register of tissue establishments laid down in Article 10(2) of the Directive. | ||
(5) The Health Products Regulatory Authority shall make information on the authorisation of importing tissue establishments available through the network of registers referred to in Article 10(3) of the Directive”. | ||
Amendment of Regulation 17 of Principal Regulations | ||
19. Regulation 17 of the Principal Regulations is amended - | ||
(a) in paragraph (1), by the substitution of “A tissue establishment, or an importing tissue establishment as the case may be,” for “A tissue establishment,”, | ||
(b) in paragraph (2), by the substitution of “tissue establishment, or on the importing tissue establishment as the case may be,” for “tissue establishment,”, | ||
(c) in paragraph (5), by the substitution of “ tissue establishment, or by the importing tissue establishment as the case may be,” for “ tissue establishment,” | ||
(d) in paragraph (6), by the substitution of “tissue establishment concerned or to its nominated representative, or as the case may be, to the importing tissue establishment concerned,” for “tissue establishment concerned”, | ||
(e) in paragraph (8), by the substitution of “ tissue establishment, or the importing tissue establishment as the case may be,” for “ tissue establishment,” | ||
(f) in paragraph (9)(b), by the substitution of “tissue establishment concerned, or the importing tissue establishment concerned as the case may be,” for “tissue establishment concerned”, | ||
(g) in paragraph (10)(b), by the substitution of “tissue establishment concerned, or the importing tissue establishment concerned as the case may be,” for “tissue establishment concerned”, and | ||
(h) in paragraph (11)(b), by the substitution of “the tissue establishment concerned, or on the importing tissue establishment concerned as the case may be.” for “the tissue establishment concerned.”. | ||
Amendment of Regulation 18 of Principal Regulations | ||
20. Regulation 18 of the Principal Regulations is amended, in paragraph (1), by the substitution of “A tissue establishment or an importing tissue establishment, as the case may be,” for “A tissue establishment”. | ||
Amendment of Regulation 19 of Principal Regulations | ||
21. Regulation 19 of the Principal Regulations is amended - | ||
(a) in paragraph (1) - | ||
(i) by the substitution of “tissue establishment or of an importing tissue establishment, as the case may be (and where appropriate, its third country supplier)” for “tissue establishment”, and | ||
(ii) in subparagraph (a), by the substitution of “tissue establishments or importing tissue establishments, as the case may be (and where appropriate, their third country suppliers) for “tissue establishments”, | ||
(b) in paragraph (2), by the substitution of “ tissue establishment sites or importing tissue establishment sites” for “tissue establishment sites”, | ||
(c) by the insertion of the following paragraph after paragraph (2): | ||
“(2A) The Health Products Regulatory Authority shall evaluate and verify the procedures and activities carried out in an importing tissue establishment and the facilities of their third country suppliers that are relevant to ensuring the equivalency of the quality and safety standards of the tissues and cells to be imported with the standards provided in the Directive and may examine any documents or other records that are relevant for this evaluation and verification.”, | ||
(d) in paragraph (3), by the substitution of “a tissue establishment, or an importing tissue establishment as the case may be,” for “a tissue establishment”, | ||
(e) in paragraph (4), by the substitution of “A tissue establishment or an importing tissue establishment” for “Any tissue establishment”, | ||
(f) in paragraph (6), by the substitution of “prescribed activities, or importing activities as the case may be, are carried out by any person on behalf of, and pursuant to a contractual arrangement with, a tissue establishment, or an importing tissue establishment as the case may be.” for “prescribed activities are carried out by any person on behalf of, and pursuant to a contractual arrangement with, a tissue establishment.”, | ||
(g) by the substitution of the following paragraph for paragraph (7): | ||
“(7) For the avoidance of doubt, it is hereby declared that the Health Products Regulatory Authority’s functions under this Regulation in relation to: | ||
(a) a tissue establishment are also applicable in the case of a tissue establishment seeking authorisation under Regulation 6; | ||
(b) an importing tissue establishment are also applicable in the case of an importing tissue establishment seeking authorisation under Regulation 6.”, and | ||
(h) by the insertion of the following paragraphs after paragraph (9): | ||
“(10) The Health Products Regulatory Authority shall, upon a duly justified request from another Member State or the Commission, provide information on the results of inspections and other control measures relating to importing tissue establishments and third country suppliers. | ||
(11) The Health Products Regulatory Authority shall, upon a duly justified request from another Member State into which imported tissues and cells are distributed after importation into the State, consider carrying out inspections or other control measures on importing tissue establishments and the activities of any third country suppliers. | ||
(12) The Health Products Regulatory Authority shall decide on the appropriate measures to take following consultation with the Member State which made a request under paragraph (11). | ||
(13) Where the Health Products Regulatory Authority undertakes an on-site inspection following a request under paragraph (11), it shall agree with the competent authority or authorities of the Member State which made such a request on whether and how that Member State shall participate in the inspection. | ||
(14) The Health Products Regulatory Authority shall decide whether or not participation in an inspection requested by the Member State that made the request under paragraph (11) is warranted. | ||
(15) Where the Health Products Regulatory Authority is of the opinion that participation in an inspection is not warranted, the decision to refuse participation under paragraph (14) shall be explained in writing to the Member State which made the request.”. | ||
Amendment of Regulation 20 of Principal Regulations | ||
22. Regulation 20 of the Principal Regulations is amended - | ||
(a) in paragraph (1), by the substitution of “tissue establishments and importing tissue establishments” for “tissue establishments” in both places that it occurs, | ||
(b) by the substitution of the following paragraph for paragraph (2): | ||
“(2) The Health Products Regulatory Authority shall maintain a publicly accessible register of tissue establishments and importing tissue establishments, specifying the activities for which they have been authorised.”, and | ||
(c) by the substitution of the following paragraph for paragraph (3): | ||
“(3) The Health Products Regulatory Authority shall assist the Commission to establish a network linking the national tissue establishment and importing tissue establishment registers.”. | ||
Amendment of Regulation 21 of Principal Regulations | ||
23. Regulation 21 of the Principal Regulations is amended by the substitution of the following definition for the definition of “relevant thing”: | ||
“ ‘relevant thing’ means - | ||
(a) any tissues or cells, including any imported tissues or cells, or | ||
(b) any article or substance used in the donation, procurement, importing, processing, preservation or storage of any tissue or cells or products manufactured from tissues and cells.”. | ||
Amendment of Regulation 22 of Principal Regulations | ||
24. Regulation 22 of the Principal Regulations is amended - | ||
(a) in paragraph (3) - | ||
(i) in subparagraph (iii), by the substitution of “premises,” for “premises, and”, and | ||
(ii) by the insertion of the following subparagraphs after subparagraph (iv): | ||
“(v) any premises owned or managed by an importing tissue establishment, or at which an importing tissue establishment carries out any importing activities, | ||
(vi) any premises of any person who carries out any importing activities on behalf of, and pursuant to a contractual arrangement with an importing tissue establishment, | ||
(vii) any premises at which books, records or other documents stored in non-legible form) relating to any importing activities are stored or kept,”, and | ||
(b) in paragraph (6)(c), by the substitution of “prescribed activity or any importing activity,” for “prescribed activity,”. | ||
Amendment of Regulation 24 of Principal Regulations | ||
25. Regulation 24 of the Principal Regulations is amended, in paragraph (1), by the substitution of the following subparagraph for subparagraph (c): | ||
“(c) a chemist, analyst or entity appointed by the Health Products Regulatory Authority,”. | ||
Amendment of Regulation 25 of Principal Regulations | ||
26. Regulation 25 of the Principal Regulations is amended, in paragraph (1), by the substitution of “6(9), 6(9A)” for “6(9),”. | ||
Amendment of Regulation 31 of Principal Regulations | ||
27. Regulation 31 of the Principal Regulations is amended by the substitution of “tissue establishments and importing tissue establishments” for “tissue establishments” in both places that it occurs. | ||
Amendment of Regulation 32 of Principal Regulations | ||
28. Regulation 32 of the Principal Regulations is amended, in paragraph (2), by the substitution of “its authorised representative, or, as the case may be, by the importing tissue establishment concerned or its authorised representative” for “its authorised representative” in both places that it occurs. | ||
Insertion of Schedules to Principal Regulations | ||
29. The Principal Regulations are amended by the insertion of Schedule 1 to 4 to these Regulations after Schedule 5. | ||
Schedule 1 | ||
Minimum requirements concerning the information and documentation to be provided by importing tissue establishment applicants when applying to be accredited, designated, authorised or licensed for the purpose of import activities | ||
When applying for an accreditation, designation, authorisation or licence for the purpose of import activities, the importing tissue establishment applicant shall, unless already provided as part of previous applications for accreditation, designation, authorisation or licensing as a tissue establishment or importing tissue establishment, provide the most up- to-date information and, for part F, documentation on the following: | ||
A. General Information on the Importing Tissue Establishment (ITE) | ||
1. Name of the ITE (Company name). | ||
2. Visiting address of the ITE. | ||
3. Postal address of the ITE (if different). | ||
4. Status of the applicant ITE: It should be indicated if this is the first application for accreditation, designation, authorisation or licensing as an ITE or, where applicable, whether this is a renewal application. Where the applicant is already accredited, designated, authorised or licensed as a tissue establishment, the TE compendium code should be provided. | ||
5. Name of the applying unit (if different from the company name). | ||
6. Visiting address of the applying unit. | ||
7. Postal address of the applying unit (if different). | ||
8. Name of the site of reception of imports (if different from the company name and applying unit). | ||
9. Visiting address of the site of reception. | ||
10. Postal address of the site of reception (if different). | ||
B. Contact Details for the Application | ||
1. Name of contact person for the application. | ||
2. Telephone number. | ||
3. E-mail address. | ||
4. Name of Responsible Person (if different from contact person). | ||
5. Telephone number. | ||
6. E-mail address. | ||
7. URL of ITE website (if available). | ||
C. Details of Tissues and Cells to be Imported | ||
1. A list of the types of tissues and cells to be imported, including one-off imports of specific types of tissues or cells. | ||
2. The product name (where applicable, in accordance with the EU generic list) of all types of tissues and cells to be imported. | ||
3. The trade name (if different to the product name) of all types of tissues and cells to be imported. | ||
4. The name of the third country supplier for each type of tissue and cell to be imported. | ||
D. Location of Activities | ||
1. A list specifying which of the activities of donation, procurement, testing, processing, preservation or storage are carried out prior to import by the third country supplier per type of tissue or cell. | ||
2. A list specifying which of the activities of donation, procurement, testing, processing, preservation or storage are carried out prior to import by sub-contractors of the third country supplier per type of tissue or cell. | ||
3. A list of all activities carried out by the ITE subsequent to import per type of tissue or cell. | ||
4. The names of the third countries in which the activities prior to import take place per type of tissue or cell. | ||
E. Details of Third Country Suppliers | ||
1. Name of third country supplier(s) (company name). | ||
2. Name of contact person. | ||
3. Visiting address. | ||
4. Postal address (if different). | ||
5. Telephone number including international dialling code. | ||
6. Emergency contact number (if different) | ||
7. E-mail address. | ||
F. Documentation to Accompany the Application | ||
1. A copy of the written agreement with the third country supplier(s). | ||
2. A detailed description of the flow of imported tissues and cells from their procurement to their reception at the importing tissue establishment. | ||
3. A copy of the third country supplier’s export authorisation certificate or, where a specific export authorisation certificate is not issued, certification from the relevant third country competent authority or authorities authorising the third country supplier’s activities in the tissue and cells sector including exports. This documentation shall also include the contact details of the third country competent authority or authorities. In third countries where such documentation is not available, alternative forms of documentation shall be provided such as reports of audits of the third country supplier. | ||
Schedule 2 | ||
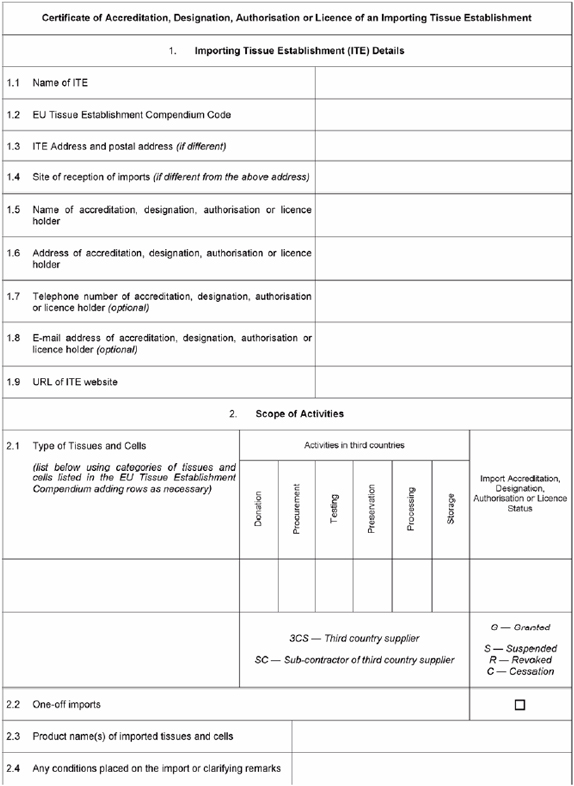
Certificate of Accreditation, Designation, Authorisation or Licence to be issued by the competent authority or authorities to importing tissue establishments | ||
| ||
| ||
Schedule 3 | ||
Minimum requirements concerning the documentation to be made available to the competent authority or authorities by tissue establishments intending to import tissues and cells from third countries | ||
With the exception of one-off imports as defined in Article 2 of this Directive which have been exempted from these documentation requirements, the applicant importing tissue establishment shall make available and, unless already provided as part of previous applications for accreditation, designation, authorisation or licensing as an importing tissue establishment or tissue establishment, shall provide when requested by the competent authority or authorities the most up-to-date version of the following documents regarding the applicant and its third country supplier(s). | ||
A. Documentation relating to the importing tissue establishment | ||
1. A job description of the Responsible Person and details of his/her relevant qualifications and training record as laid down in Directive 2004/23/EC; | ||
2. A copy of the primary label, repackage label, external package and transport container; | ||
3. A list of relevant and up-to-date versions of standard operating procedures (SOPs) relating to the establishment’s import activities including SOPs on applying the Single European Code, reception and storage of imported tissues and cells at the importing tissue establishment, management of adverse events and reactions, management of recalls and traceability from donor to recipient. | ||
B. Documentation relating to the third country supplier or suppliers | ||
1. A detailed description of the criteria used for donor identification and evaluation, information provided to the donor or donor family, how consent is obtained from the donor or donor family and whether the donation was voluntary and unpaid or not; | ||
2. Detailed information on the testing centre(s) used by third country suppliers and the tests performed by such centres; | ||
3. Detailed information on the methods used during the processing of the tissues and cells including details of the validation for the critical processing procedure; | ||
4. A detailed description of the facilities, critical equipment and materials and criteria used for quality control and control of the environment for each activity carried out by the third country supplier; | ||
5. Detailed information on the conditions for release of tissues and cells by the third country supplier or suppliers; | ||
6. Details of any sub-contractors used by the third country suppliers including the name, location and activity undertaken; | ||
7. A summary of the most recent inspection of the third country supplier by the third country competent authority or authorities including the date of the inspection, type of inspection and main conclusions; | ||
8. A summary of the most recent audit of the third country supplier carried out by, or on behalf of, the importing tissue establishment; | ||
9. Any relevant national or international accreditation. | ||
Schedule 4 | ||
Minimum requirements concerning the contents of written agreements between importing tissue establishments and their third country suppliers | ||
With the exception of one-off imports as defined in Article 2 of this Directive which have been exempted from these requirements, the written agreement between the importing tissue establishment and the third country supplier shall contain at least the following provisions. | ||
1. Detailed information on the specifications of the importing tissue establishment aimed at ensuring that the quality and safety standards laid down in Directive 2004/23/EC are met and the mutually agreed roles and responsibilities of both parties in ensuring that imported tissues and cells are of equivalent standards of quality and safety; | ||
2. A clause ensuring that the third country supplier provides the information set out in Annex III B to this Directive to the importing tissue establishment; | ||
3. A clause ensuring that the third country supplier informs the importing tissue establishment of any suspected or actual serious adverse events or reactions which may influence the quality and safety of tissues and cells imported or to be imported by the importing tissue establishment; | ||
4. A clause ensuring that the third country supplier informs the importing tissue establishment of any substantial changes to its activities, including any revocation or suspension, in part or in full, of its authorisation to export tissue and cells or other such decisions of non-compliance by the third country competent authority or authorities, which may influence the quality and safety of tissues and cells imported or to be imported by the importing tissue establishment; | ||
5. A clause guaranteeing the competent authority or authorities the right to inspect the activities of the third country supplier, including on-site inspections, should it wish to do so as part of its inspection of the importing tissue establishment. The clause should also guarantee the importing tissue establishment the right to regularly audit its third country supplier; | ||
6. The agreed conditions to be met for the transport of tissues and cells between the third country supplier and importing tissue establishment; | ||
7. A clause ensuring that donor records relating to imported tissues and cells are kept by the third country supplier or its sub-contractor, in line with EU data protection rules, for 30 years following procurement and that suitable provision is made for their retention should the third country supplier cease to operate; | ||
8. Provisions for the regular review and, where necessary, revision of the written agreement including in order to reflect any changes in the requirements of the EU quality and safety standards laid out in Directive 2004/23/EC; | ||
9. A list of all standard operating procedures of the third country supplier relating to the quality and safety of imported tissues and cells and a commitment to provide these on request. | ||
| ||
GIVEN under my Official Seal, | ||
5 February 2019 | ||
SIMON HARRIS, | ||
Minister for Health. | ||
Explanatory Note | ||
(This note is not part of the instrument and does not purport to be a legal interpretation) | ||
These Regulations give effect to Commission Directive (EU) 2015/566 of 08 April 2015 implementing Directive 2004/23/EC as regards the procedures for verifying the equivalent standards of imported tissues and cells. | ||
The Regulations provide procedures for verifying that tissues and cells imported from a third country (i.e. from outside the European Union), meet standards of quality and safety equivalent to those laid down in Directive 2004/23/EC, in order to ensure a high level of protection of human health. | ||
The Regulations amend the European Communities (Quality and Safety of Human Tissues and Cells) Regulations 2006 ( S.I. No. 158 of 2006 ). | ||
The Regulations may be cited as the European Communities (Quality and Safety of Human Tissues and Cells) (Amendment) Regulations 2019. | ||
2 OJ No. L 93, 9.4.2015, p.56. |